Healthcare data management for smarter patient care and innovation
Connect your most critical data for smarter care, faster research, and compliance you can trust with Semarchy’s data management platform built for healthcare and life sciences. Streamline operations, power AI initiatives, and achieve better health outcomes with unified, reliable data.
Better healthcare starts with better data
Uncover your data’s value to improve patient care, reduce operational inefficiencies, and accelerate R&D breakthroughs. Unified data helps healthcare organizations enhance patient experiences and meet evolving medical demands.

Enhance patient experiences and health outcomes
Deliver personalized care, improve safety, and enhance drug efficacy and trial success by unifying data across multiple systems, departments, and locations.

Optimize healthcare operations and costs
Eliminate data silos, automate workflows, and maximize the use of resources, from personnel to medical equipment and supplies, to cut costs and boost operational productivity.

Accelerate medical research and development
Fast-track healthcare innovation by streamlining data processes and delivering rapid insights, shortening time-to-market for new drugs, treatments, and therapies.

Gain real-time health insights
Leverage real-time analytics to improve decision-making, allocate resources effectively, adapt to challenges, and enhance organizational agility without burdening IT teams.
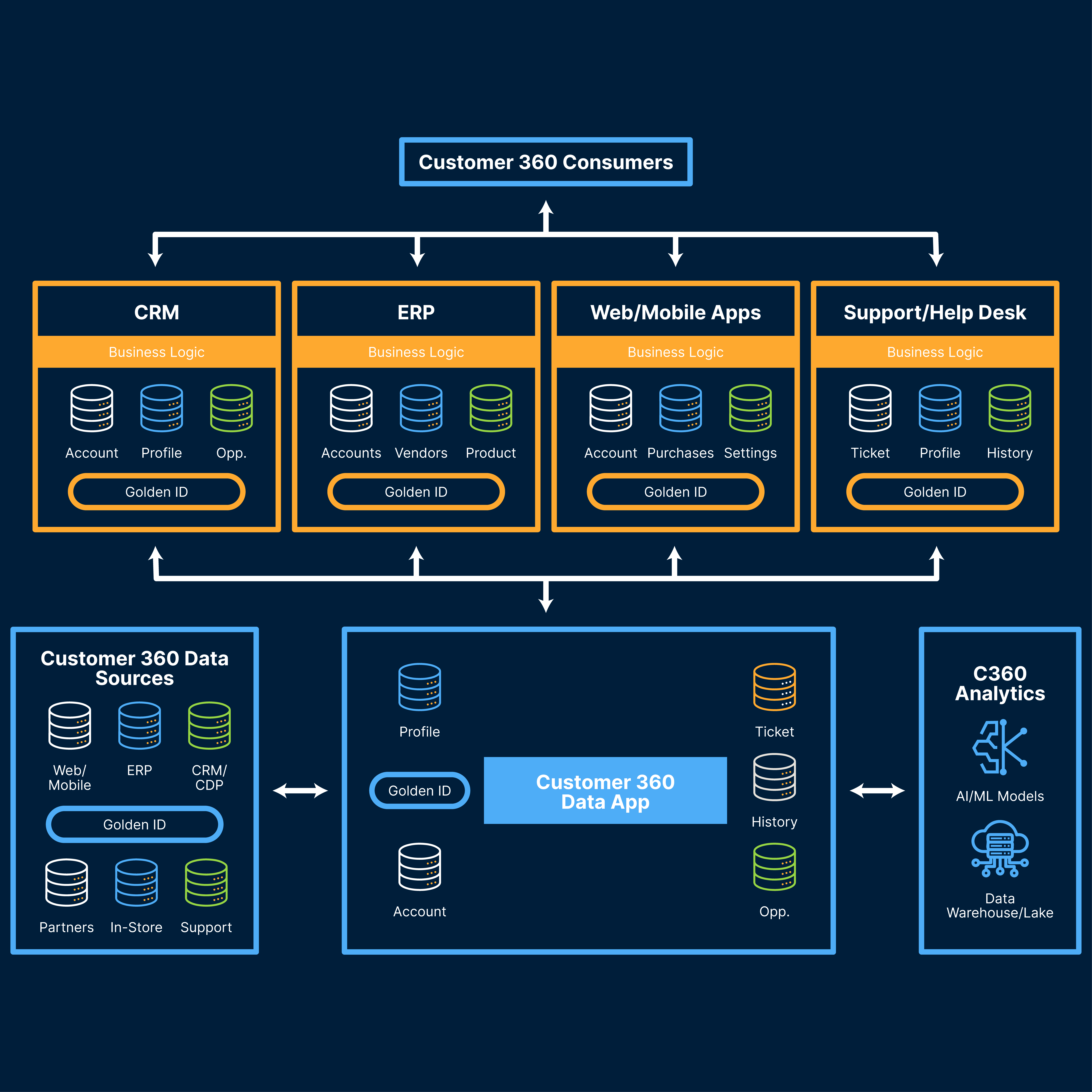
Build 360° patient and stakeholder profiles
Create a complete, real-time view of patients, providers, and other key stakeholders. Enable tailored treatments, stronger patient-provider relationships, reduced care gaps, and improved health outcomes with a single source of truth.
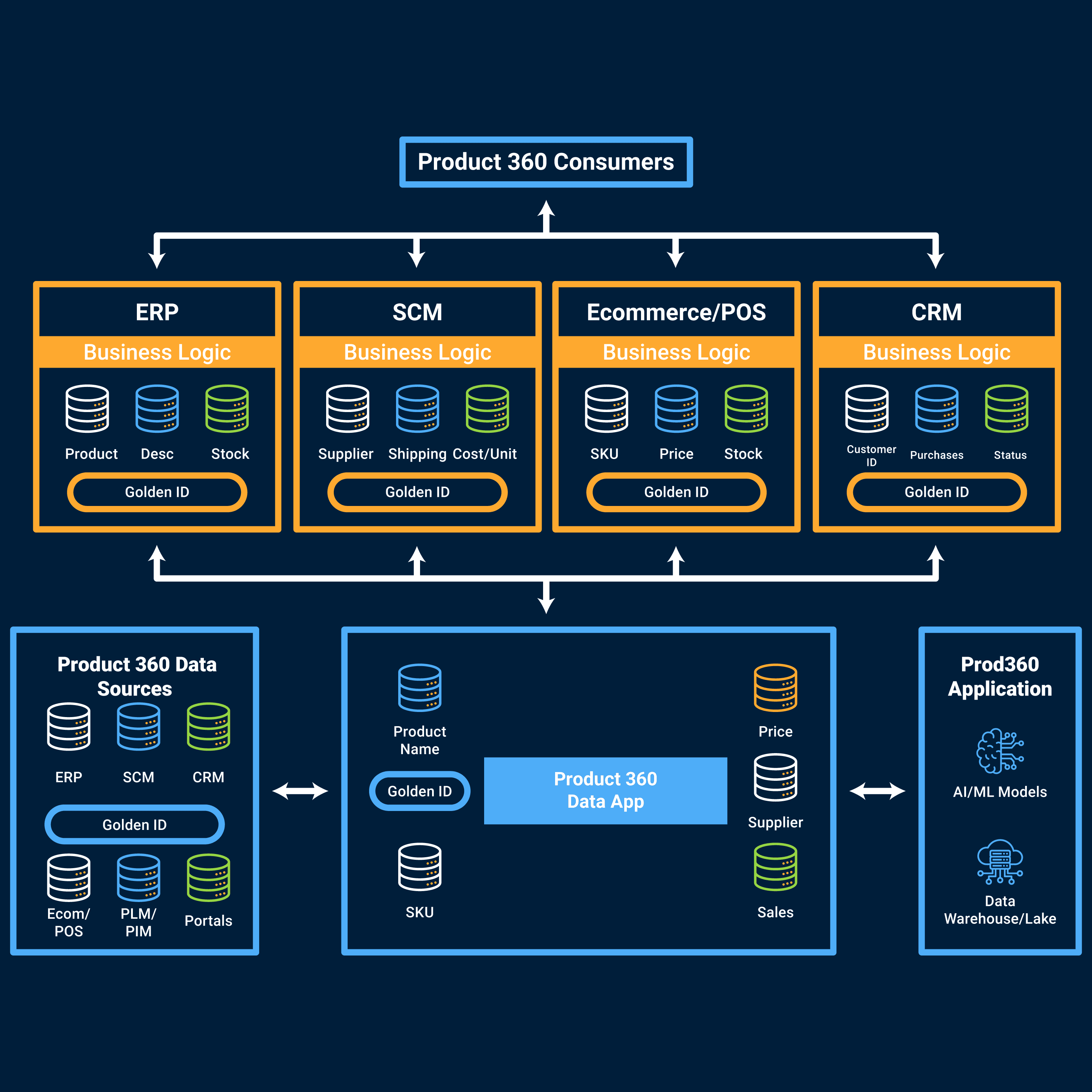
Optimize product and supply lifecycles
Track and manage the lifecycle of medical supplies, pharmaceutical products, and equipment. From inventory to compliance, get accurate data and visibility across the entire supply chain operation.
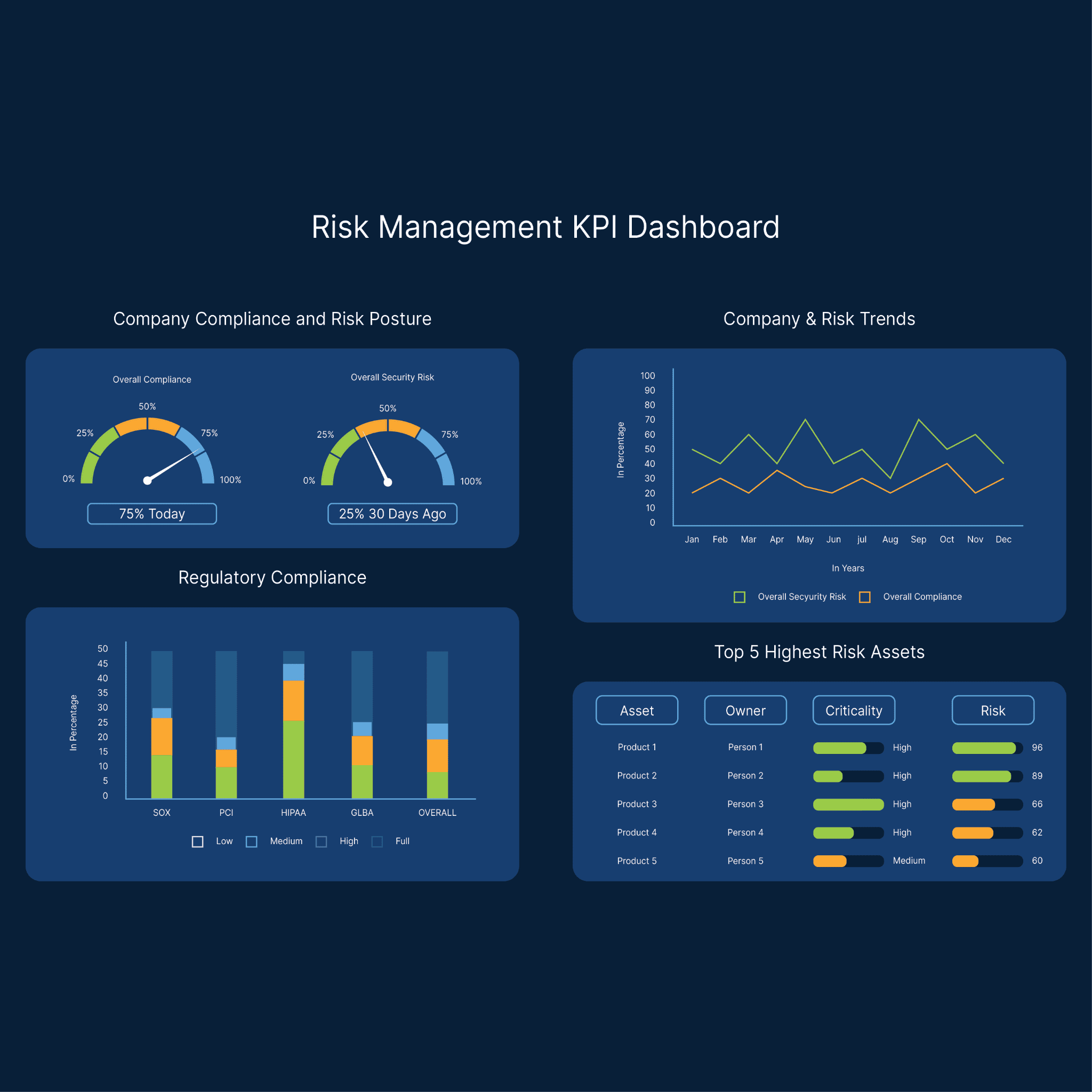
Comply with regulations and automate reporting
Meet HIPAA compliance and adapt to evolving healthcare regulations. With built-in governance, automated reporting, and high-quality data, you can streamline audits, address recalls and fixes quickly, and protect patient data at every step.
Trusted by enterprise data leaders around the world
Our healthcare and life sciences customers value Semarchy’s rapid deployment, expert support, and ability to drive sustainable improvements in patient care, research, and compliance now and into the future.

2024 Gartner Peer Insights Customers’ Choice
Customers have named Semarchy their preferred MDM provider six years in a row (and counting).
See whyConsolidated master records managed
We’ve successfully handled complex data initiatives across a range of industries and domains.
See our impact
SoftwareReview Emotional Footprint Awards
Semarchy received the highest possible ranking from users, with a +92 Net Emotional Footprint.
Learn moreReady to master your healthcare data?
Semarchy is your partner in healthcare and life sciences innovation. From operational efficiency to improved health outcomes, we can help you achieve ROI in less than 12 weeks with a fully functioning unified healthcare data management platform.